Lithium is a soft, silvery-white alkali metal having the symbol Li. It is the third element in the periodic table and is the lightest among the metals [1]. Like all alkali metals, lithium is highly reactive and flammable and will even react spontaneously with water. As a result, Lithium is never found in its elemental, metallic form, but only in (usually ionic) compounds, such as pegmatitic minerals. As a whole, the Earth’s crust contains approximately 20 parts per million of lithium, and the oceans contain 0.17 parts per million; the atmosphere contains only trace amounts.
The common method of extracting lithium is hard rock mining where lithium can be extracted from lithium minerals found in igneous rocks (chiefly spodumene) [2]. In this mining process, the mineral is extracted from open pit mines and then extracted by roasting it. This process requires a large amount of water and is usually completed near rivers or lakes, or the water is diverted to the mines. In 2020, the highest amount of lithium was sourced mainly from the hard rock mining process and this type of mining is mostly used in Australia subcontinent.
The other conventional method involves the extraction of lithium from underground brine reservoirs below the surface of dried lake beds [3]. In order to extract lithium minerals from the ground, miners will start by drilling a hole in the ground and pumping in brine into the hole and then leave the brine to rest on the surface. As the brine rests, the liquid evaporates leaving behind a dense collection of minerals. It takes roughly 12-18 months for everything to evaporate off before the minerals can be collected in a certain region. In comparison with hard rock mining, this extraction procedure needs more water to extract a comparable amount of lithium. [4]. The use of electro-dialysis and electrochemical intercalation has been proposed to extract lithium from seawater (which contains lithium at 0.2 parts per million), but it is not yet commercially viable.
The other process of extracting lithium is the geothermal wells in which lithium chlorides can be carried out to the surface and using a simple filtration process, lithium can be separated [5]. Although this process has a smaller environmental footprint, the reserves are more limited as compared to hard rock mining and underground brine reservoirs. In recent time, the extraction of lithium using geothermal water is mostly found in the Germany and the USA.
Lithium mining at the end of the day is still a mining process, which means it does have some environmental impact. [4]. Therefore, to reduce that environmental impact, we always recommend recycling your lithium batteries when they reach end-of-life.

Figure 1: Spodumene (lithium aluminum silicate), a commercial source of lithium [1].
Considering the importance of lithium for the modern world, it would be worthwhile to review the current production and reserves of lithium. As per the report of the United States Geological Survey [6], Australia is the highest producer of lithium in 2019 with a percentage of 52.9% of global production. On the other hand, Chile, China, Argentina, Zimbabwe, and the United States of America contribute 21.5%, 9.7%, 8.3%, 2.1%, and 1.2 % of the global lithium production. However, the world is only producing a tiny fraction of its lithium reserves. Based on 2019 production levels, known global lithium reserves would last more than 200 years. In 2019, the world’s Top 5 lithium reserves by country were Chile, Australia, Argentina, China, and the United States of America that had 55.5%, 18.1%, 6.5%, and 4.1% of the world’s total lithium reserve. Country-wise production and reserve of Lithium as reported by the United States Geological Survey in 2019 is illustrated in Figures 2 and 3, respectively.

Figure 2: Country wise producers of lithium in 2019 [1].

Figure 3: Country wise reserve of lithium in 2019 [1].
In recent time, the global market for lithium is increasing day by day and it has found its application in many industries such as glasses, ceramics, and pharmaceuticals. Between 2008 and 2018 alone, annual production in the major producing countries rose from 25,400 to 85,000 tons. Lithium is a special type of metal as it is light and soft and exhibits so low density that it can float on water. The main feature of this metal is that it has the lowest melting point and the highest boiling point i.e. it can withstand a wide range of temperatures. However, the main application of this material is in the automation industries, to be specific, as a battery power source for electric vehicles.
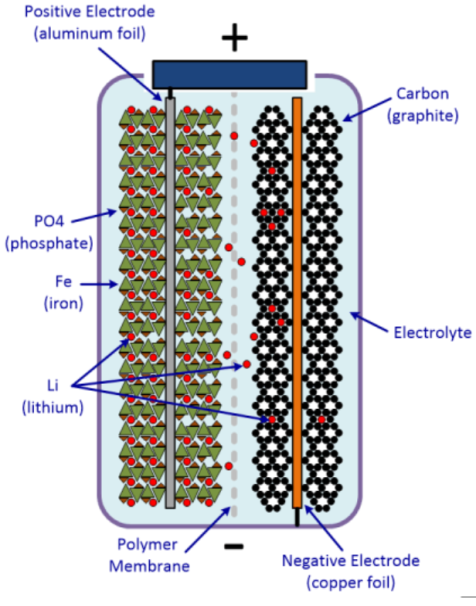
Lithium-ion batteries are drastically more efficient and sustainable than any other battery technique. In another article, we listed the Advantages of Lithium Iron Phosphate Batteries over Lead-Acid Batteries. They also have significant energy density compared to cost-effective alternatives, which makes them perfect for electric cars. Moreover, lithium is also used in the batteries of laptops and cell phones, as well as in any other battery-powered devices. For battery applications, lithium is used as the electrode and electrolyte material in disposable lithium batteries and in rechargeable lithium-ion batteries.
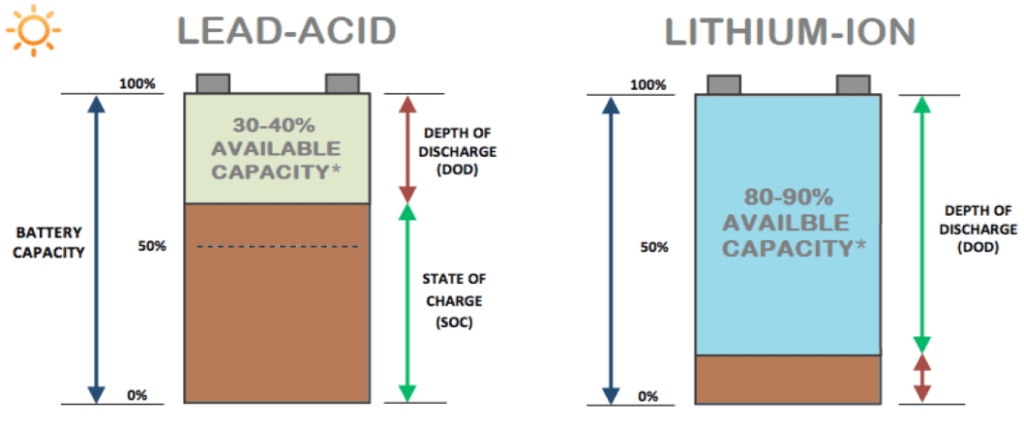
Although lithium is an important material considering its application in industrial sectors, the infrastructure of sourcing and mining the lithium minerals is not as cost-effective as conventional lead-acid batteries. But the long term life expectancy of LiFePO4 has proven to be a more cost-effective total cost of ownership solution over lead-acid equivalents.
References
[1] https://www.usgs.gov/centers/nmic/lithium-statistics-and-information
[2] B.Tadesse, F. Makuei, B. Albijanic, L. Dyer, “The beneficiation of lithium minerals from hard rock ores: A review”, Minerals Engineering, vol. 131, Jan 2019, pp. 170-184.
[3] J. Sterba, A. Krzemień, et al., “Lithium mining: Accelerating the transition to sustainable energy”, Resources Policy, vol. 62, Aug 2019, pp. 416-426.
[4] W. Liu, D.B. Agusdinata, S.W. Myint, “Spatiotemporal patterns of lithium mining and environmental degradation in the Atacama Salt Flat, Chile”, International Journal of Applied Earth Observation and Geo information, vol. 80, Aug 2019, pp. 145-156.
[5] S. Chen, Z. Chen, Z. Wei, J. Hu, Y. Guo, T. Deng, “Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water”, Chemical Engineering Journal, vol. 410, April 2021, 128320.
[6] https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-lithium.pdf