A battery is defined as a device with electrochemical cells to power electrical devices such as lights, mobile phones, and electric vehicles. The positive terminal of a battery is called the cathode, the negative terminal is called an anode. A schematic diagram of battery is shown in Figure 1. The anode terminal is the source of electrons that will flow through an external load to the cathode i.e. positive terminal [1]. The cell consists of concentric alternating layers of the negative and positive electrode materials between which separator layers are situated. The cell is then filled with electrolyte to allow ion conduction.

Figure 1: Schematic diagram of a battery [1].
Challenges: With the availability of different electrochemical materials, the lithium based battery system can be designed to a specifical application regarding voltage level, SOC, lifetime, and safety. The electrochemical couples can also be used to design batteries as per the available energy. The manufacturing of large a cell requires a good quality electrode and active material usage. Electrodes are coated on a metal current collector foil in a composite structure of active material that requires careful control of colloidal chemistry, adhesion, and solidification.
Fundamentals: In early days, lithium cobalt oxide (LiCoO2) was used to manufacture the lithium ion battery because of its ability to release lithium ion, creating large vacancies. During the charge, the released lithium ions travel from the positive terminal to negative terminal through the electrolyte. When the battery feeds an electric load i.e. during discharging, the lithium ions came back from the negative electrode to the positive electrode. At each electrode, the ion either maintains its charge and intercalates into the crystal structure occupying interstitial sites in existing crystals on the anode side or reoccupies a vacant site in the cathode that formed when the lithium ion left that crystal. While transferring the ion, the host matrix gets reduced or oxidized, which releases or captures an electron.
Cathode Materials: The material used to make the cathode electrode is built as a source of lithium ions. Since a carbon electrode is used as the anode terminal in lithium battery, it does not contain any lithium. Hence, the positive terminal must be manufactured in such a way that it can release a vast amount of lithium ions during the battery operation. The most common cathode material is LiCoO2 that was used for years [2]. However, the LiCoO2 presents many disadvantages. The battery has a core temperature of 40–70°C and may be susceptible to some low-temperature reactions. But, at a temperature range of 105–135°C, it is very reactive and an excellent oxygen source for a safety hazard called a thermal runaway reaction, in which highly exothermic reactions create temperature spikes and accelerate rapidly with the release of extra heat.

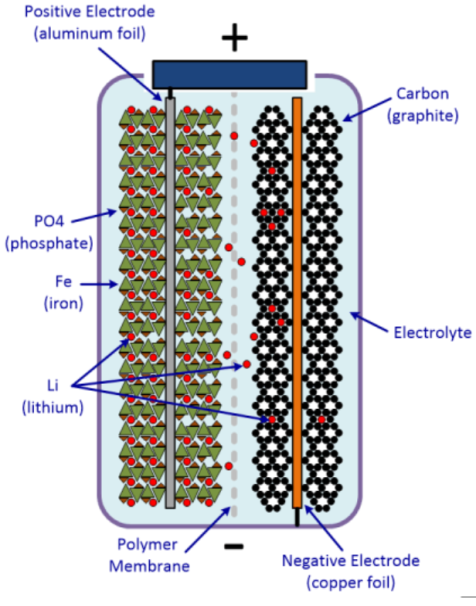
Figure 2: Schematic diagram of LiFePO4 battery.
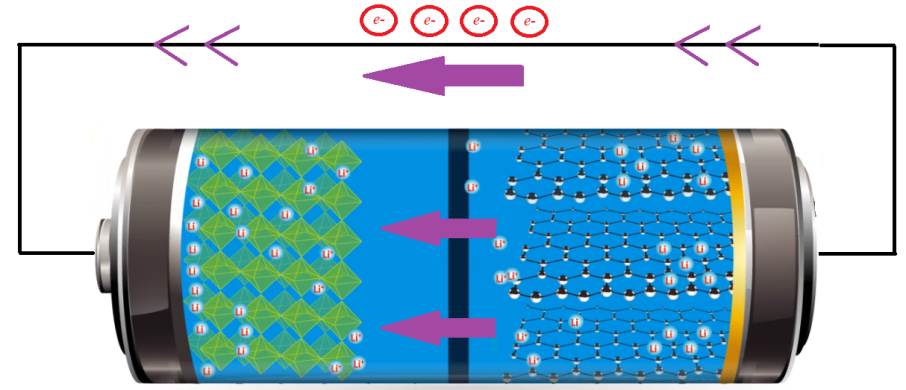
To alleviate these challenges, LiFePO4 finds its application as a replacement for LiCoO2 as a cathode material. In LiFePO4 batteries, the iron and phosphate ions form grids that loosely trap the lithium ions as shown in Figure 2. During the charging of the cell, these loosely trapped lithium ions easily get pulled to the negative electrode through the membrane in the middle. The membrane is made of a type of polymer having lots of tiny little pores for the lithium ions to pass through easily. On the negative side, there is a lattice made of carbon atoms, which can trap and hold those lithium ions that cross over. Discharging the battery does the same thing in reverse: As electrons flow away through the negative electrode, the lithium ions once again go on the move, through the membrane, back to the iron-phosphate lattice. They are once again stored on the positive side until the battery gets discharged again. Although LiFePO4 batteries exhibit capacities in the range of 120–160 Ah/kg at 3.5–3.7 V and energy density of up to 600 Wh/kg, bare LiFePO4 materials suffer from many disadvantages, such as low conductivity and sluggish diffusion rate of Li+ ions, which becomes a chief barrier to commercialization [3]. This problem was overcome by reducing the particle size, coating the LiFePO4 particles with conductive materials such as carbon nanotubes [4]. Moreover, olivine-type LiFePO4 is considered as one of the most promising cathode materials for Li ions batteries owing to its high theoretical capacity, low cost, high operating voltage and no environmental pollution, and accordingly, it is being commercialized for its application in energy sectors and electric vehicles.
Anode Materials: Anode materials form the negative electrode of LiFePO4 batteries, which act as the host where they reversibly allow lithium-ion intercalation during and de-intercalation during discharge cycles. The anode materials must have low irreversible loss, high efficiency, a fast lithium-ion diffusion rate, high conductivity, and high specific capacity etc. In earlier days, petroleum cokes were used in lithium-ion batteries to form the anode electrode. In recent time, natural or synthetic graphite is used as shown in Figure 2. However, with the advancement in technology, as a substitute of graphite, Lithium titanate (LTO) has become a promising candidate for anode material [5]. LTO operates on a stable voltage of ≈1.5 V vs. Li+/Li, which avoid electrolyte decomposition and the safety issues presented by the use of carbon that tends to form lithium dendrite, causing an initial loss in capacity, safety issues, and loss of critical electrode performance. Moreover, as compared to other anode materials namely Si, SiOx, SiO2, Fe2O3, and Co3O4, LTO presents superior structural stability with no structural or volume changes during the charging and discharging cycle. Hence, LTO can be most suitable for LiFePO4 battery applications that require a high rate, long cycle life, and high efficiency, and better safety. Again, LTO as a cost-effective solution is years from mass production and integration to the consumer markets.
Electrolytes: The electrode and the separator must be filled up with an electrolyte during the manufacturing process of LiFePO4 batteries [6]. An incomplete filling can cause a negative impact on electrochemical performance, life cycle of the battery and safety issues. The main function of the electrolyte is to make way for transporting the positive lithium ions from the cathode electrode to the anode electrode. The most commonly used electrolyte is comprised of lithium salt, such as LiPF6 in an organic solution.
Battery Management System: Depending upon the applications of lithium battery, large number of battery cells may be connected in series to increase their voltage range or otherwise in parallel to increase its current capacity. Each battery when operating in series has a slightly different capacity due to manufacturing tolerances and environmental conditions. After several charge/discharge cycles, an individual cell can have lower performance and capacity, so the capacity of the entire battery pack will be reduced. In such context, Battery Management System helps to maintain cell balancing of a LiFePO4 battery bank by distributing an equal charge amongst the battery cells to make it safe, reliable, and cost efficient [7]. The voltage difference among cells connected in series can be caused by a SOC imbalance, a total capacity imbalance or an internal impedance imbalance. Using proper microprocessor based control algorithms; battery management systems offers precise measurement and estimation of the State of Charge, Depth of Discharge, State of Health and protection from overcharging and deep discharging, which in turn maintains each cell of the battery pack within its safe operating range.
Cost Reductions: Due to higher cost of lithium ion batteries, electric vehicles are much costlier than internal combustion engines based automobile. The current cost of commercial LiFePO4 batteries is $400–500/kWh and it has been targeted by the US Department of Energy to reduce the cost to $125/kWh of usable energy [8]. The most suitable way to reduce the battery cost is by increasing the energy density. Further cost reduction is also possible through optimization of manufacturing processes. In recent time, the replacement of N-Methylpyrrolidone (NMP) by water is a tremendous opportunity to reduce cost in the production of lithium ion batteries since the cost of water is negligible compared to that of NMP and added advantages are that water is not flammable and environmental friendly [9]. Further cost reductions will be achieved through greater knowledge of transport mechanisms and electrode architecture implications for electrochemical performance.
LiFePO4 batteries have tremendous potential as energy storage devices in the automation industry and for higher penetration of intermittent renewable energy sources. However, a higher cost is always an obstacle but falling a little more each month.
References
[1] D. Goonetilleke, J. C. Pramudita, M. Hagan, et al., “Correlating cycling history with structural evolution in commercial 26650 batteries using in operando neutron powder diffraction”, Journal of Power Sources, vol. 343, 2017, pp. 446-457.
[2] C. Daniel, D. Mohanty, J. Li, D.L. Wood, “Cathode materials review. Review on Electrochemical Storage Materials and Technology”, AIP Conference Proceedings, vol. 1597, 2014, pp. 26–43.
[3] J. Li, C Rulison, J. Kiggans, C. Daniel, D.L. Wood, “Superior performance of LiFePO4 aqueous dispersions via corona treatment and surface energy optimization”, Journal of the Electrochemical Society, vol.159, no. 8, 2012, pp. A1152–A1157.
[4] J. Li, B.L. Armstrong, J. Kiggans, C. Daniel, D.L. Wood, “Lithium ion cell performance enhancement using aqueous LiFePO4 cathode dispersions and polyethyleneimine dispersant”, Journal of the Electrochemical Society, vol. 160, no. 2, 2013, pp.A201–A206.
[5] A. Purwanto, S. U. Muzayanha, C. S. Yudha, et al., “High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery”, Applied Sciences, vol. 10, Oct 2020, 7135
[6] M. Montanino, S. Passerini, G.B. Appetecchi, “4 – Electrolytes for rechargeable lithium batteries (Rechargeable Lithium Batteries From Fundamentals to Applications)”, Woodhead Publishing Series in Energy,2015, pp. 73-116.
[7] B. Li, D. Yang, J. Liu, M. Chen, Z. Lu, “An Optimal Strategy of Balancing for LiFePO4 Battery in Battery Energy Storage System”, Applied Mechanics and Materials, vol. 341-342, 2013, pp. 1286-1293.
[8] https://www.hydrogen.energy.gov/index.html
[9] J. Li, Y. Lu, T. Yang, D. Ge, D.L. Wood, and Z. Li, “Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System”, iScience, vol. 23, issue 5, 2020 May, 101081.