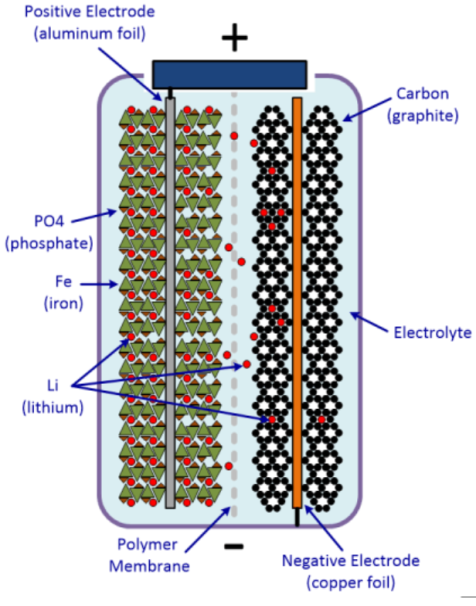
Like any other battery, Lithium Iron Phosphate (LiFePO4) battery is made of power-generating electrochemical cells to power electrical devices. As shown in Figure 1, the LiFePO4 battery consists of an anode, cathode, separator, electrolyte, and positive and negative current collectors. The positive terminal of a battery is called the cathode, whereas the negative terminal is termed as the anode. The anode terminal acts as the source of lithium ions. The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. The movement of the lithium ions creates free electrons in the anode and as a result, electrons will flow through an external circuit to the cathode i.e. positive terminal, and accordingly, a current will flow from the positive terminal to the negative terminal when an electric load is connected across the battery [1]. The cell consists of concentric alternating layers of the negative and positive electrode materials between which separator layers are situated. The cell is then filled with electrolyte to allow ion conduction.
The cathode terminal must be manufactured in such a way that it can release a vast amount of lithium ions during the battery operation. The most common cathode material is LiCoO2, however, there are some disadvantages associated with the material [2]. As a result, LiFePO4 finds its application as a replacement for LiCoO2 [3]. In recent times, the anode terminal is made from natural or synthetic graphite. However, with the advancement in technology, Lithium Titanate (LTO) has become a promising candidate for anode material as a substitute for graphite. The most commonly used electrolyte is comprised of lithium salt, such as LiPF6 in an organic solution.

The working principle of LiFePO4 regarding the charging and discharging cycles is discussed in the following section:

Charging State: The positive electrode i.e. the cathode is constructed from lithium-iron-phosphate. The iron and phosphate ions form grids where the lithium ions are loosely trapped. As shown in Figure 2, when the battery is getting charged, these lithium ions get pulled through the membrane and reach the negative graphite electrode that can trap and hold these cross over lithium ions [1]. The membrane is made of a type of polymer (plastic) that has lots of tiny little pores to make it easy for the lithium ions to pass through. The battery will be fully charged when all the positive lithium ions available in the cathode terminal reach the anode terminal and are stored between layers of graphene accordingly.
The typical charging profile of a LiFePO4 battery is illustrated in Figures 3 and 4 [4]. To make the analysis easier, it is assumed that 4 single cell batteries are connected in series to convert the voltage of the battery pack to approximately 12 Volts. The charging of LiFePO4 batteries can be classified into two stages as discussed below [4]:


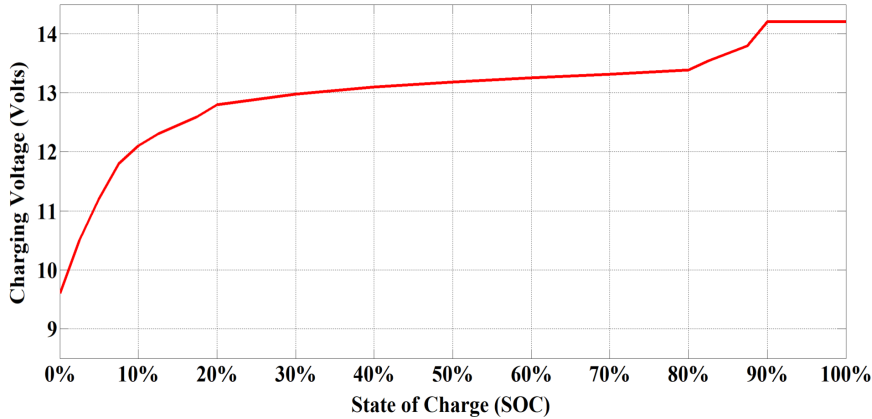
- Constant current charge: In the first stage of charging, the current is kept constant at a charge rate of 0.5C as shown in Figure 4, indicating that the battery will get charged at a charge rate of half of its capacity. For example, a charge rate will be kept constant at 100Amp for charging a battery with 200Ah capacity. As illustrated in Figure 3, the charging voltage of the battery will slowly rise during the constant current charging period and the value of voltage will reach the ‘absorb’ Voltage i.e. 14.4 V.
- Saturation Charge: Once the battery is 90% charged i.e. the absorb voltage is reached, the battery is entered into a second stage of charging known as the saturation charge. At this point, the battery voltage is kept constant and the current will steadily fall. As soon as the current drops to around 5% – 10% of the battery’s Ah rating, 100% state of charge (SOC) is reached.
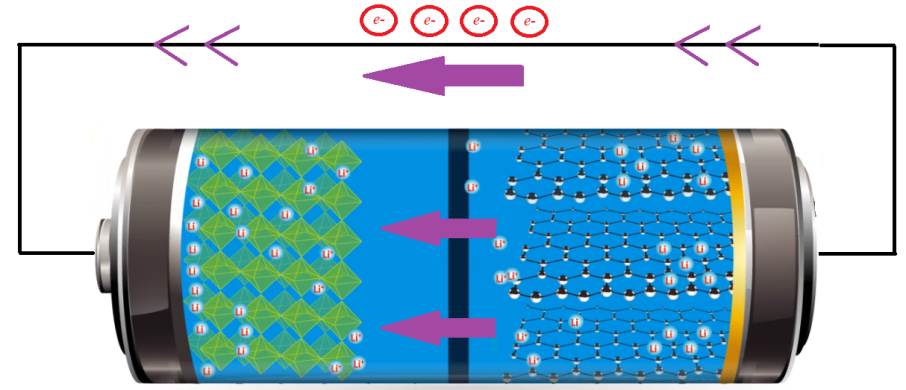
Discharging State: As discussed earlier, during the charging cycle of a LiFePO4 battery, the released positive lithium ions from the positive electrode move to the negative electrode through the electrolyte and remain stored there [1]. When all the available lithium ions reach the negative terminal, the battery is said to be completely charged. As illustrated in Figure 5, when the charged battery is connected across an electrical load, the positive ions move back to the positive electrode from the negative electrode through the separator. At the same time, electrons flow through the outer circuit, resulting in a current flow through the electrical load circuit and the battery will discharge its stored energy. Due to the presence of an insulating barrier (i.e. the separator), the electrons cannot flow through the electrolyte. When the battery is fully discharged, all the lithium ions have moved back to the lithium-iron-phosphate electrode.


The discharge cycle of a 12 Volt LiFePO4 (4*single-cell battery voltage) battery is shown in Figure 6 [4]. This figure indicates the variation of the discharge voltage corresponding to different values of SOC. It is clear from the discharge cycle of the LiFePO4 battery that the curve remains almost flat during the discharge from 100% SOC to nearly 20% SOC. Therefore, the external electrical loads can be supplied with a constant voltage magnitude irrespective of the charge level of the battery, which makes it advantageous in comparison with a lead-acid battery. In other types of lithium-ion batteries, it is not a good idea to go below 20% of SOC, but LiFePO4 batteries can be discharged all the way down to 0% for many cycles. However, the battery cycle life can be hampered if the battery is continuously discharged to 0% SOC.
The charging and discharging of lithium-ion batteries is the key to their operations and long-term performances. Therefore, it is essential to ensure that the batteries are charged and discharged in an appropriate manner [5]. In such context, the following safety measurements such as charging current (maximum value is 0.8C), charging temperature (in the range between 00C to 450C), discharging current protection, over-voltage protection, over-charge protection, reverse polarity protection and over-discharge protection must be considered. To safely manage the charging and discharging of LiFePO4 batteries, a battery management system is integrated into the battery packs. If a user forgets to unplug the charging system, this system will automatically stop charging the battery to prevent overcharge and on the other hand, this system will also ensure the battery is not discharged beyond the specified limit.
References:
[1] D. Deng, “Li-ion batteries: basics, progress, and challenges”, Energy Science and Engineering; vol. 3, no. 5, 2015, pp. 385–418
[2] D. Goonetilleke, J. C. Pramudita, M. Hagan, et al., “Correlating cycling history with structural evolution in commercial 26650 batteries using in operando neutron powder diffraction”, Journal of Power Sources, vol. 343, 2017, pp. 446-457.
[3] J. Li, B.L. Armstrong, J. Kiggans, C. Daniel, D.L. Wood, “Lithium ion cell performance enhancement using aqueous LiFePO4 cathode dispersions and polyethyleneimine dispersant”, Journal of the Electrochemical Society, vol. 160, no. 2, 2013, pp.A201–A206.
[4] M. Verasamy, M. Faisal, P. J. Ker, M.A. Hannan, “Charging and Discharging Control of Li-Ion Battery Energy Management for Electric Vehicle Application”, International Journal of Engineering & Technology, vol. 7, no. 4.35, 2018, pp. 482-486
[5] B. Mao, C. Liub, K. Yang, “Thermal runaway and fire behaviors of a 300 Ah lithium ion battery with LiFePO4 as cathode”, Renewable and Sustainable Energy Reviews, vol. 139, Apr 2021, 110717.